A different treatment experience backed by a robust body of evidence
FDA-approved indications1
years of postmarketing experience1
years of total clinical use2*
clinical trials across indications1,2†
patient-years of worldwide cumulative exposure2‡
*Human trials initiated in July 1998. First patient, first dose in RA December 1998. Clinical studies investigated patients with RA, CD, PsA, and other diseases, as well as healthy subjects.
†Includes approved indications for RA, CD, PsA, AS, PSO, and nr-axSpA as well as other completed and ongoing research.
‡Patient exposure was estimated using the available sales data from September 1, 2007 to February 28, 2022 for the cumulative time interval. The exposure of CIMZIA was calculated using the following formula: Patient-years=([total mg of product distributed]/[monthly maintenance dose])/12 months in year.
CIMZIA is the only Fc-free anti-TNF3-7
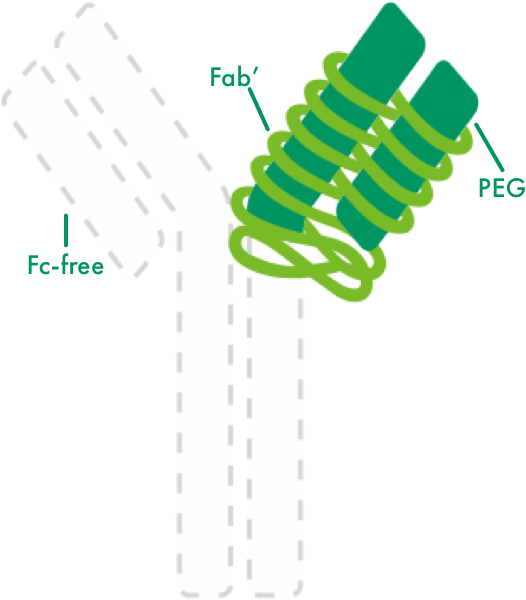
CIMZIA does not contain an Fc portion, which is present in a fully formed antibody.
A monovalent TNF inhibitor monoclonal antibody fragment, CIMZIA neutralizes TNF-alpha at low concentrations with a high affinity.1
PEGylation changes the physical and chemical properties of the biochemical molecule.8
- Increases bioavailability of CIMZIA9,10
- Extends half-life of the antigen-binding fragment1,5
- Increases drug stability and retention time, thereby allowing a reduced dosing frequency5,8
Clinical relevance of in vitro and in vivo data is unknown.
The images do not imply comparable safety, efficacy, or indication.
Fab, fragment antigen binding; Fc, fragment crystallizable; PEG, polyethylene glycol; IgG1, immunoglobin G1.




